
 |
||||||||||||||||
Platelet-surface interactions: priming of platelets by upstream agonists for downstream adhesion
- Concept: The transient nature of agonist-induced platelet adhesion and activation has largely been ignored in the design of vascular implants. The objective is to study how platelets interact with blood-contacting biomaterials by taking into account the history of upstream platelet- agonist contacts.
- One of the original observations indicated that the upstream history matters in downstream adhesion (Fig 1).
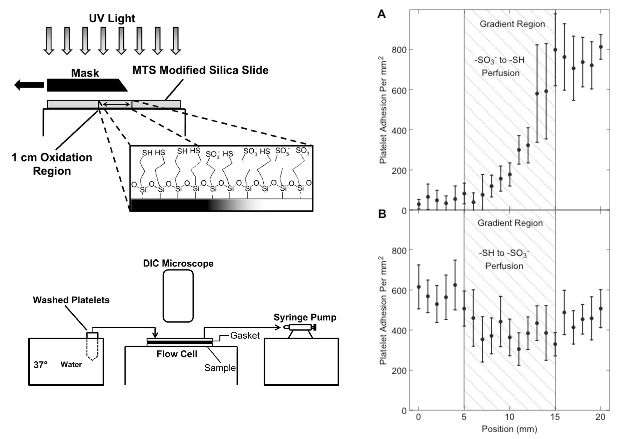
Fig 1. A thiol to sulfonate surface gradient was generated by UV oxidation of surface thiols, then exposed to 10% platelet free plasma, followed by the washed platelet perfusion through two identical flow channels but in opposite directions. Adsorbed fibrinogen on the -SH side of the gradient primed platelets for the adhesion to the sulfonate end of the gradient downstream (panel B), however, this effect was absent whan the flow direction was reversed. (from Corum and Hlady, Biomaterials 31 (2010) 3148–3155).
- Concept: Is it possible that any upstream agonists 'prime' platelets for downstream adhesion and activation? That question was answered in the next set of experiments. A flow cell was designed to contain an upstream agonist patch with covalently immobilized fibrinogen and downstream capture patch for adhesion of 'primed' platelets. Inbetween the two patches was an inert PEO coating (Nexterion, Schott) that was also passivated by adsorbed albumin. Washed platelets suspension has been flowed over the agonist region and platelets captured downstream were counted. When the priming agoinst was blocked by a policlonal antibody, or was absent, downstream capture numbers dropped by more than 50% (Fig 2).
Fig 2. Flow cell used in upstream- downstream studies (width = 0.5 cm, height = 0.025 cm, wall shear rate, = 100 1/s). Flow of platelets (5 min @ 20 ml/h) leads to the encounter of platelets with the ‘priming’ region (1 cm long). The ‘primed’ platelets flowed downstream where they were captured by the downstream capture region (100% fibrinogen coverage, 1 cm long)(left panel). Blocking the fibrinogen ‘priming’ region with an anti-fibrinogen polyclonal antibody attenuates the downstream adhesion response (right panel). (from Corum and Hlady, Biomaterials 33 (2012) 1255-1260).
- Downstream capture of platelets was agonist dose-dependent: - increasing the surface density of upstream agonist also increased the number of 'primed' platelets captured downstream (Fig 3).
Fig 3. Downstream platelet adhesion from whole blood was agonist dose-dependent. An increasing surface concentration of covalently attached fibrinogen (Fgn) in the ‘priming’ region resulted in increased adhesion to the downstream capture region (100 % fibrinogen coverage) (from Eichinger and Hlady, unpublished).
- Platelet spreading on mixed fibrinogen-albumin coating was limited by the size of fibrinogen micro-islands in the coating (Fig 4).
Fig 4. Platelet adhesion to covalently immobilized fbrinogen patterns at coverages of 20, 50, and 85%. The nonprinted region is blocked with covalently immobilized albumin. The top and middle rows contain fuorescent images (green) of fibrinogen patterns and DIC images of platelet adhesion and spreading, respectively. (A) The bottom row includes superimposed images, where at 20% platelets rarely spread and when they do their size is strictly limited by the size of the underlying fbrinogen pattern. (B) At 50%, platelets are able to spread, but their morphology almost strictly depends on the shape of the underlying fibrinogen pattern. (C) At 85%, platelets are able to spread over inert albumin regions, and the morphology is not necessarily dependent on the underlying fibrinogen pattern (from Corum, Eichinger, Hsiao and Hlady, Langmuir 2011, 27, 8316–8322).